Post Preview
Key Takeaways
- Clinical trials are essential for developing new medical treatments and improving patient care.
- Advancements like decentralized trials and AI integration are making trials more accessible and efficient.
- Real-world evidence is increasingly influencing clinical trial designs and healthcare decisions.
The Role of Clinical Trials in Medical Advancements
Clinical trials play a foundational role in medical progress, offering the critical data required to assess the safety and effectiveness of new medications and therapies. These trials allow physicians and scientists to determine whether interventions truly benefit patients before they are widely adopted in clinical practice. Innovations such as vaccines for infectious diseases and targeted cancer drugs owe their introduction to the thorough vetting processes of clinical studies. Understanding the phases of these trials and their significance can help patients and providers make more informed healthcare decisions. For a deeper look at clinical research, see this detailed guide to clinical trial phases explained.
Each phase of a clinical trial builds on data collected previously, moving from small groups of healthy volunteers to larger, more diverse patient populations. This progression helps researchers identify potential side effects, optimal dosing, and real-world effectiveness. As medicine advances, the role of trials becomes even more crucial, ensuring that breakthroughs reach patients safely and efficiently.
Clinical trials also create standards of care by rigorously evaluating which treatments work best for certain diseases or patient subgroups. This not only accelerates the adoption of innovation but also protects the public from unproven or unsafe therapies. Over the past decade, increased collaboration among academic centers, biotech companies, and regulatory agencies has streamlined the path from discovery to widespread use.
Beyond new medications, clinical trials are essential for surgical innovations, diagnostics, and preventive care strategies. They provide a standardized framework for comparing interventions and adapting recommendations in light of emerging evidence.
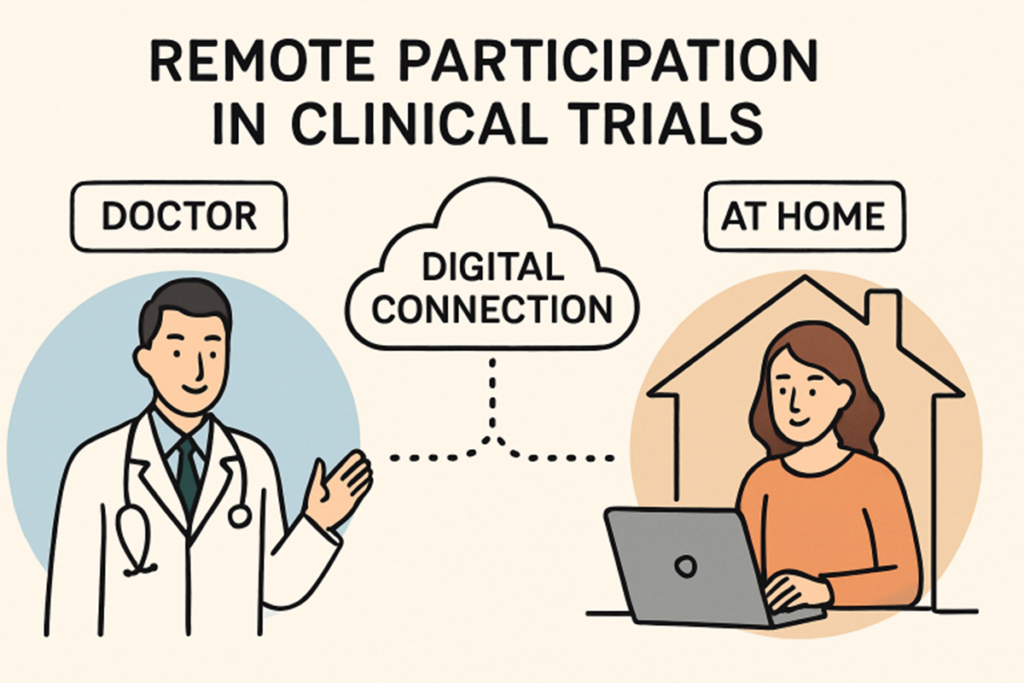
Decentralized Clinical Trials: Bringing Research to Patients
Traditional clinical trials have often required patients to travel to designated study sites, posing challenges of time, distance, and cost. Decentralized clinical trials (DCTs) address these barriers by utilizing digital technologies, allowing participants to join studies remotely. This shift significantly improves accessibility, enabling individuals from rural or underserved areas to contribute to critical research without relocating or traveling long distances.
DCTs also increase the diversity of clinical trial populations. By reducing logistical barriers, more patients from different backgrounds, ethnicities, and socioeconomic groups can participate. Radicle Science, a health tech company based in San Diego, has pioneered remote research platforms that support fully virtual studies. This model is making clinical trials both more inclusive and representative of real-world populations, helping bring efficient new treatments to more people.
Artificial Intelligence: Revolutionizing Trial Matching
One of the most significant challenges in clinical research is matching patients to the right trials. Artificial intelligence (AI) and large language models are transforming this process by parsing extensive health data and matching eligibility criteria with patient records. This not only speeds up the recruitment phase but also makes it more accurate, ensuring that people who most need innovative treatments have better access.
Recent studies highlight the vast potential of AI-driven trial matching, particularly in fields like oncology, where patient profiles and treatment options are complex. By automating the identification process, AI reduces manual labor and improves match precision.
Real-World Evidence: Informing Trial Designs
Traditionally, clinical trials have relied on carefully controlled environments and highly specific patient cohorts. However, real-world evidence (RWE) involves analyzing health data from everyday clinical practice, electronic health records, and patient registries. This approach helps researchers design studies that better reflect actual patient experiences and circumstances. Integrating RWE makes trial findings more applicable to the populations that will ultimately use the treatments.
Physicians are increasingly recognizing the importance of RWE in clinical research. By blending observational data with randomized controlled trials, healthcare systems can develop more pragmatic guidelines and improve patient outcomes worldwide. The adoption of RWE is a significant step forward in bridging the gap between clinical research and the needs of everyday patients.
Addressing Global Inequities in Trial Participation
Despite technological advancements and greater awareness, major disparities in access to clinical trials continue to affect patients worldwide. Factors such as geographic location, local research infrastructure, and socioeconomic status can all limit participation. Efforts to reduce these inequities involve building research capacity in underserved regions, providing remote access through decentralized trials, and increasing outreach to ensure that people from diverse backgrounds are represented in medical research.
Pragmatic Trials: Bridging Research and Practice
Pragmatic clinical trials evaluate treatments in settings that mirror everyday clinical realities, rather than idealized conditions. By focusing on real-world effectiveness and practical outcomes, pragmatic studies provide invaluable data for doctors and policymakers. These trials translate directly into changes in clinical guidelines and reimbursement policies, ensuring that innovation reaches the patients who need it most in a timely and effective manner.
Patient-Centric Approaches: Empowering Participants
Modern clinical research increasingly centers on participants’ experiences, preferences, and feedback. Patient-centric approaches involve engaging with patients at every stage, from study design to implementation and follow-up. By soliciting and integrating patient input, trials become more relevant and respectful of participant needs, ultimately leading to greater satisfaction and better medical outcomes.
Conclusion
Clinical trials sit at the heart of medical innovation, determining which treatments are safe, effective, and appropriate for broad use. Ongoing advancements in decentralized models and artificial intelligence, as well as the integration of real-world evidence, are making these studies more accessible, efficient, and applicable to the general population. Continued progress will depend on addressing disparities in access and prioritizing patient experience, so every individual can benefit from the advances of modern medicine.